Chapter 18 Renal tubular acidosis
18.1 Classification of RTA
type II (pRTA) = impaired reabsorption of HCO3
type I (dRTA) = inability to secrete H+:
- complete = systemic acidosis
- incomplete = no systemic acidosis
- complete = systemic acidosis
type IV = hypoaldosteronism (+/- impaired ammoniagenesis from hyperK)
type III (mixed) = CA inhibition
RTA in CKD:
- HCMA when GFR < 30
- WGMA when GFR < 15
18.1.1 Pathogenesis of hypokalaemic RTA
Normally, the tubular threshold for HCO3 is around 24 - 26 mM, so that plasma [HCO3] is maintained in that range.
In dRTA, there is no change in the tubular threshold for HCO3 but due to a failure of distal acidification, urine pH never falls even in the face of systemic acidosis.
In pRTA, there is a reduction in the apparent Tm (and hence tubular threshold) for HCO3. As a consequence, in mild systemic acidosis, there is renal bicarbonate wasting, maintaining an alkaline urine. However, as the acidosis becomes more profound and [HCO3] drops below the tubular threshold, there is no tubular HCO3 loss and - because distal acidification remains intact - the urine can be acidified.
(These mechanisms were established by Edelman and colleagues in children with RTA in the late 1960s.)
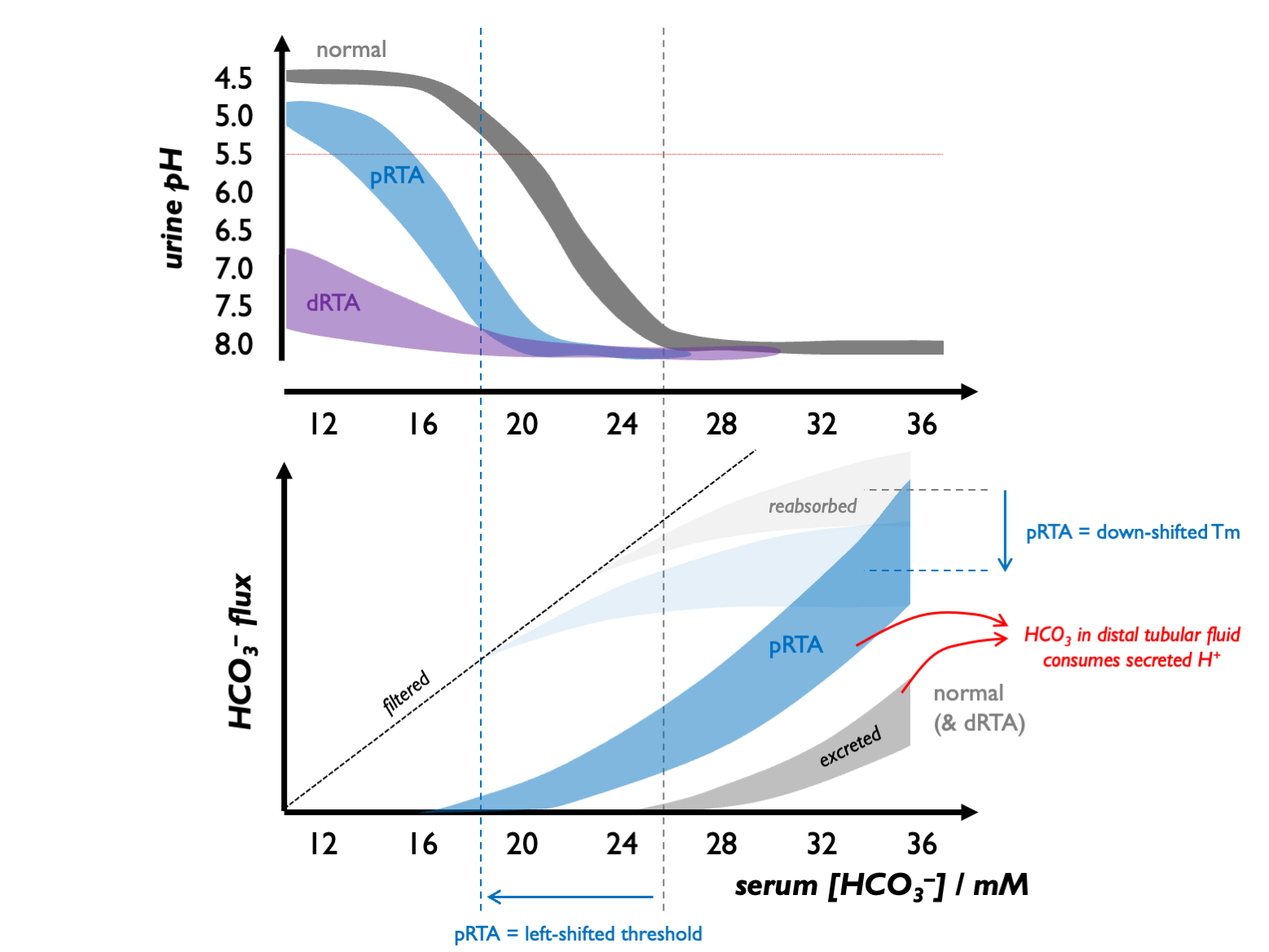 Therefore:
Therefore:
in dRTA: urine pH is never low (always well above 5.5); a negative acid balance can never be achieved - hence the severe skeletal phenotype
in pRTA: urine pH may be alkaline or acid - depending on how profound the systemic acidosis is; negative acid balance can be achieved, avoiding a very severe skeletal phenotype
Furthermore:
in dRTA: relatively modest HCO3 supplementation is required in dRTA (in the order of 1 mmol per kg per day to regenerate that lost to the buffering of non-volatile acid)
in pRTA: absolutely massive HCO3 supplementation - above 10 mmol per kg per day - would be required to drive systemic [HCO3] into the normal range; this might end up being counter-productive if the associated bicarbonaturia drives excessive K+ and Na+ loss
18.1.2 Pathogenesis of hyperkalaemic RTA
Two mechanisms account for type IV RTA:
reduced mineralocorticoid activity in the distal nephron, meaning that there is less electrogenic Na+ reabsorption (through ENaC) and a diminished electrical force driving H+ and K+ secretion;
hyperkalaemia per se impairs ammoniagenesis in the PCT
18.1.3 Associations
- dRTA CaP stones (alkaline urine, hypocitraturia, hypercalciuria)
- nephrocalcinosis (in inherited forms particularly)
- osteoporosis
18.1.4 Investigation of suspected RTA
UAG & FENaHCO3
urine pH (never < 5.5 in dRTA)
in suspected pRTA
- tubular reabsorption of phosphate (TRP = 100 - FEPO4) < 85 %
- glycosuria, aminoaciduria, LMW proteinuria
in suspected dRTA
- furosemide-fludrocortisone (FF) test (failure to achieve pH < 5.3 , 3 – 4 hrs after 40 – 80 mg / 1 mg)
- UCa > 4 mg / kg / day (or spot UCa/Cr > 0.2) in type I RTA
- USS / KUB (medullary nephrocalcinosis in type I RTA)
- urinary citrate (low in type I RTA; high in type II / IV RTA)
kidney biopsy (to detect subclinical TIN)
Test urinary RBP (retinol binding protein) as the most sensitive marker of proximal renal tubular dysfunction - good in sarcoid, Sjogren’s etc. Good for diagnosis and for tracking disease activity.
USS more sensitive than CT for nephrocalcinosis in the context of hypoparathyroidism, but CT more specific (Boyce JCEM 2013). Plain films very insensitive. Therefore prefer USS for screening (but consider CT for verification).
18.1.5 Causes
| PRTA (ISOLATED) | inherited | inherited |
| acquired | topiramate | |
| PRTA (WITH FANCONI) | inherited | NBCe1A (AR with ocular abnormalities) |
| Wilson’s | ||
| cystinosis | ||
| fructose intolerance | ||
| Dent disease | ||
| mitochondrial cytopathies | ||
| myeloma | ||
| LCDD | ||
| LCFS is κ in 96 %, | ||
| amyloid | ||
| Sjogren’s | ||
| other TIN | ||
| allograft rejection | ||
| tenofovir | ||
| lamivudine | ||
| aminoglycosides | ||
| outdated tetracyclines | ||
| cisplatin | ||
| valproate | ||
| lenalinomide | ||
| Pb | ||
| Hg | ||
| Cd | ||
| aristolochic acid | ||
| DRTA | inherited | AEI (AR) |
| H-ATPase B1 (AR with SNHL) | ||
| H-ATPase A4 (AR) | ||
| chronic pyelonephritis | ||
| chronic TIN | ||
| obstructive uropathy | ||
| sickle cell | ||
| allograft rejection | ||
| hypergammaglobulinaemia | ||
| SLE | ||
| Sjogren’s | ||
| chronic active hepatitis | ||
| PBC | ||
| Li+ | ||
| amphoterocin | ||
| toluene | ||
| hyperPTH | ||
| idiopathic hypercalciuria | ||
| MSK | ||
| TYPE IV | low renin | DM |
| NSAIDs | ||
| CNIs | ||
| β– | ||
| Addison’s | ||
| CAH | ||
| ACEi / ARB | ||
| heparin | ||
| ketoconazole | ||
| TIN | ||
| sprionolactone / amiloride | ||
| trimethoprim | ||
| TYPE III | inherited | CAII (AR with osteopetrosis / cerebral calcification) |
| topiramate (CA inhibition) |
Sjogrens classically causes a dRTA (but can also cause pRTA if interstitial nephritis). Autoimmune dRTA is Sjogrens in 80 - 90%. Subclinical tubular involvment in 30% Sjorens. Often associated with hypocitruria and low-grade features of Fanconi syndrome. Treat CIN with MMF or pred / RTX. Treat dRTA with K citrate. Autoantibodies to H+-ATPase or bicarb exchanger.
Mitochondrial cytopathy: raised CK, abnormal number of microchondria (EM).
18.2 Fanconi syndrome
In adults, usually acquired:
- tenfovir and other drugs (antivirals, fumaderm for Psoriasis)
- heavy metals
- LCCD
- post-Tx
Check for:
- LMWH proteinuria (e.g. retinol binding protein; high uPCR with a negative dip)
- uricosuria
- phosphaturia
- renal glycosuria
LMW proteinuria is the most sensitive test (because megalin is the most energy-sensitive process and therefore most vulnerable to becoming disrupted). Glycosuria appears last (least energy-sensitive process).