Chapter 4 Acid-base & chloride
4.1 Acid-base homeostasis
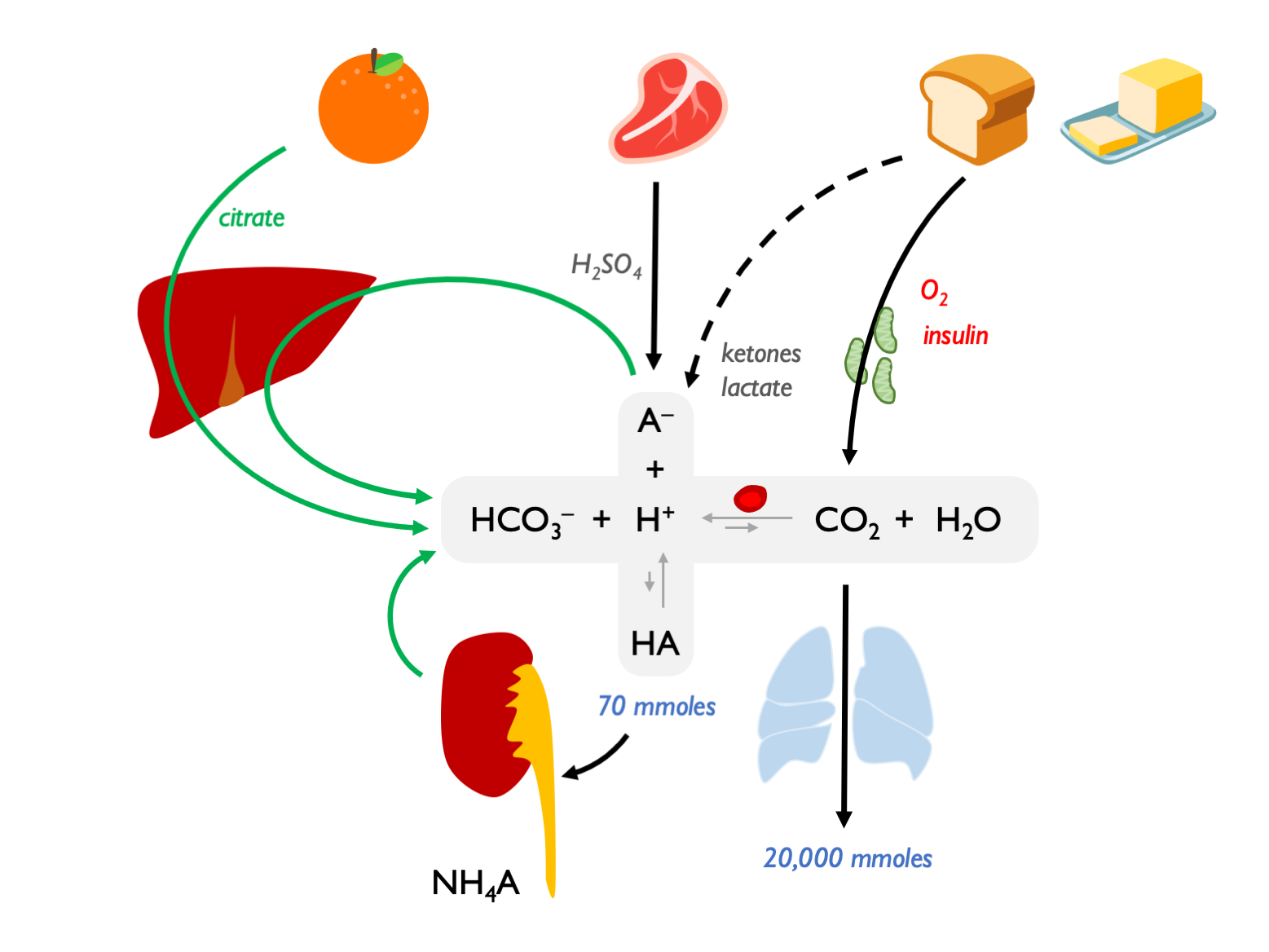
Summary of the dietary sources of acids and alkali and the role of the kidney in generating bicarbonate (to aid the excretion of non-volatile acid):
4.3 Adaptation
The relationship between pH, PaCO2 and HCO3 is described by the Henderson-Hasselbalch equation:
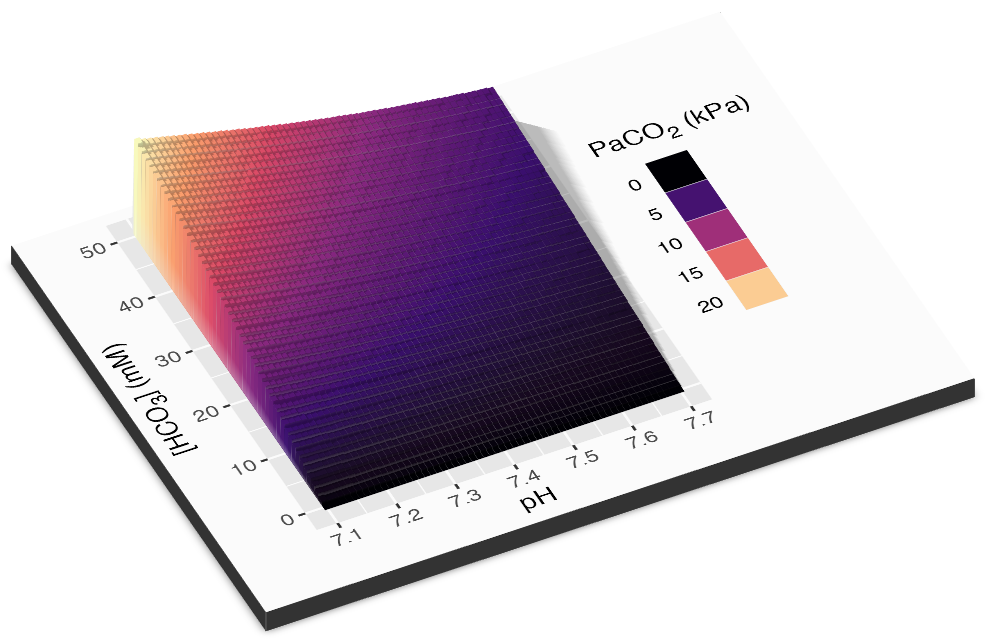
This relationship can be visualised in 3D…
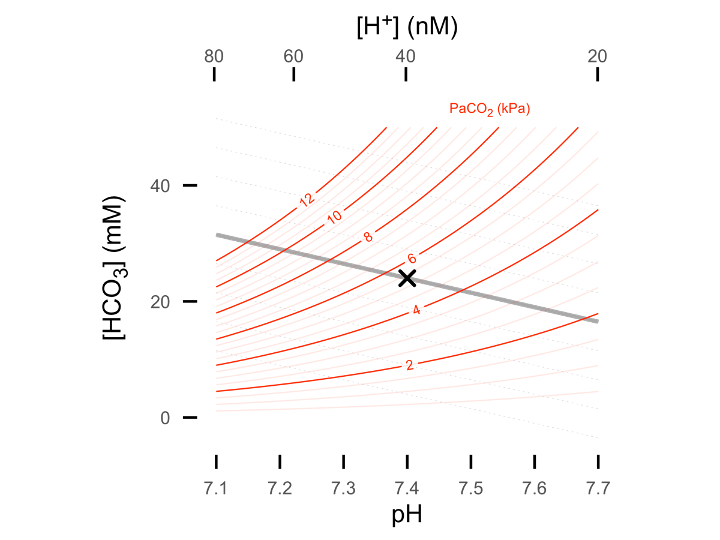
…or 2D in the form of a Davenport plot (shown below) - or alternatively as a Siggard-Anderson plot (pH vs. PaCO2 with BE nomogram lines).
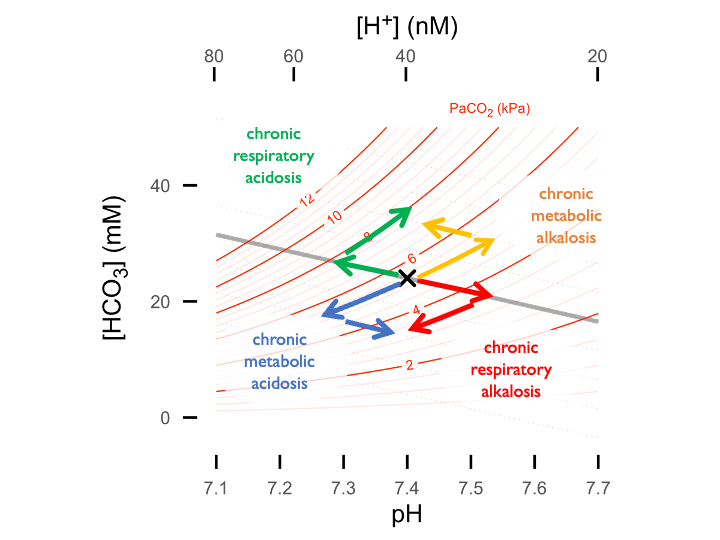
Metabolic adaptation to respiratory alkalosis:
- acute (5 – 10 mins) HCO3 16 – 18 mM buffering by serum HCO3
- chronic (2 – 3 days) HCO3 12 – 15 mM renal excretion of HCO3
Respiratory adaptation:
- for each mM HCO3 < 25 PaCO2 should drop by… 0.16 kPa (1.2 mmHg)
- for each mM HCO3 > 25 PaCO2 should rise by… 0.08 kPa (0.6 mmHg)
- Winter’s formula: PaCO2 (mmHg) = (1.5 × HCO3) + 8 +/- 2 (in metabolic acidosis)
4.4 Renal control of acid-base homeostasis
Renal net acid excretion is determined by urinary titratable acid, ammonium and bicarbonate:
In the face of a daily acid load, the main job of the kidneys is to regenerate HCO3 for the ECF in order to replace that lost through buffering of non-volatile acids. It does this through mechanisms that keep urine pH ~ 6 to minimise risks of uric acid precipitation (in acid urine) or calcium phosphate precipitation (alkaline urine).
The three key processes are:
- reabsorption of filtered HCO3
- excretion of titratable acid (H2PO4) = net HCO3 reabsorption
- excretion of ammonium (glutamine > NH3 + H+ > NH4+) = net HCO3 reabsorption
4.4.1 Normal renal response to chronic acidosis
The normal response of the kidneys to chronic acidosis is to upregulate urinary NH4 excretion. This was illustrated in historical acid-loading studies:
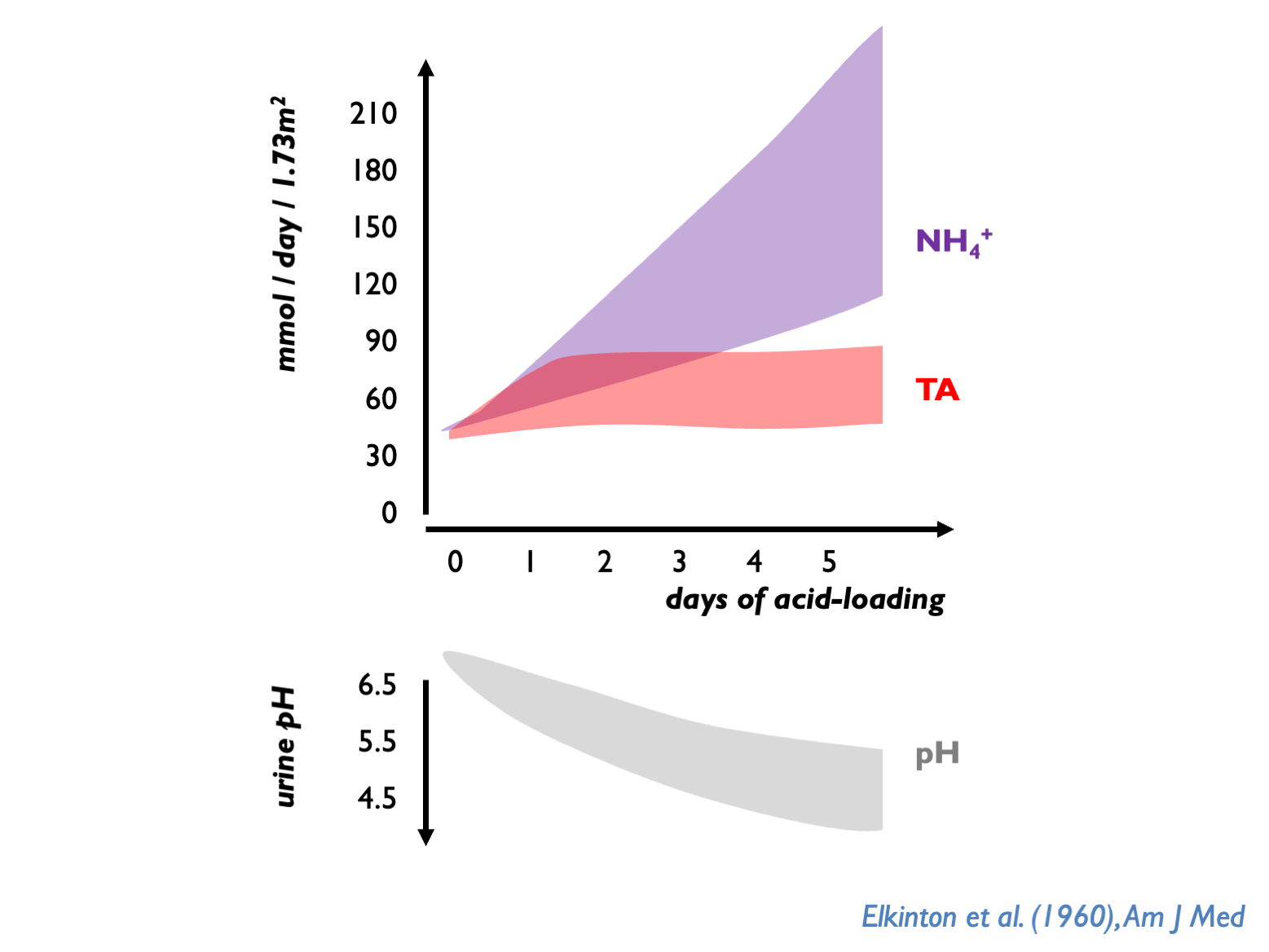
The preferential use of NH4 over TA is probably an evolutionary defense against kidney stones. NH4 tends to keep upH around 6.0 - thus avoiding very acid urine (risk of uric acid precipitation) or very alkaline urine (risk ofCaHPO4 precipitation).
Normally, kidney has to dispose ot 70 mmol non-volatile acid per day through TA and NH4. uNH4 therefore normally 30 – 40 mmol per day; up to 100 - 200 mmol per day after acid-loading (Kamel & Halperin, KI Reports 2021; Uribarri, AJKD 2022). uNH4 (directly-measured) higher on Western than plant-based diets (Uribarri AJKD 2022).
4.4.2 Renal response to alkalosis
An alkalosis will correct automatically as HCO3- exceeds the Tm.
There is an apparent Tm for HCO3-, set close to 25 mM but variable and influenced by various factors (GFR, luminal pH, hormonal factors etc.) For example, during volume depletion, stimulation of Na reabsorption (NHE activity) will increase HCO3- re-absorption, so leading to an increase in the apparent Tm for HCO3-. Under normal circumstances, FEHCO3 is < 0.1% (Lote).
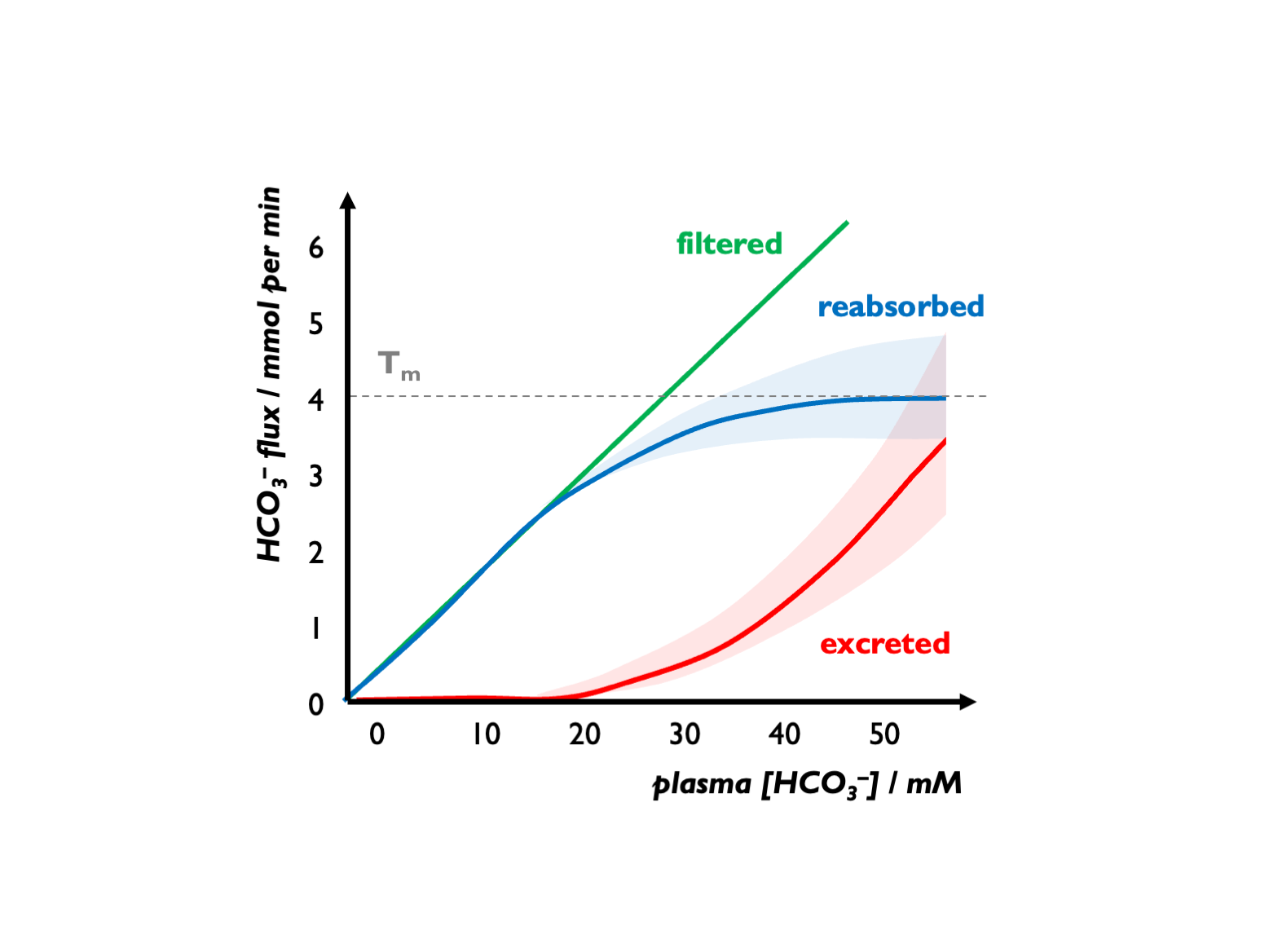
In alkalosis, bicarbonaturia can drive cation loss (e.g. hypokalaemia)